Subcutaneous Immunoglobulin (SCIg) Therapy
Both IVIg and SCIg are purified immunoglobulin type G (abbreviated IgG) – biologics made from the pooled plasma of thousands of healthy human donors.3,4 The biologics consist of mostly IgG antibodies and trace amounts of IgA, IgM, and other plasma proteins.6 IgRT is primarily prescribed to treat patients with PI.4
There are several SCIg products on the market today, differing by their concentration, stabilizers, and infusion specifics.7 The number of products available, coupled with the flexibility of the SCIg regimen, has made SCIg a popular choice for PI patients worldwide.7
SCIg can either be self-infused at home (after proper training) or administered in a medical setting with a healthcare provider, in either case using a needle set, designed explicitly for SC infusions. There are several SCIg infusion sets available with needle sizes and lengths that range considerably, to match specific patient need and preference. Studies have shown, however, that if misused, the shorter needles can cause drug leakage and irritation at the infusion site.7
SCIg tubing sets also vary depending on how many administration sites and infusion pumps are being used. Tubing sets are available with anywhere from 1 to 5 leg branches attached to a single trunk.7 The patient’s preferred tubing set is connected to either a syringe or portable infusion pump. Some infusion pumps use an IV bag (called a cassette), which is filled with the SCIg solution and connected to the pump. Others hold a 50 mL syringe that is placed in either an automatic mechanical pump or electrical pump, set to a programmed infusion rate.
Usually, SCIg is administered under the skin on the belly, inner thighs, or outer buttocks.8 Sometimes, the upper arm areas can be used as well.7 (See Figure 15.) Where to stick the needle (called the site) and how many sites will be needed depend on how much SCIg (called the volume) is going to be infused that day. Infusion sites are also dependent on the patient, who may choose where it is most comfortable to place a needle during an infusion. Sometimes, only one site is required; other times, many sites are needed to complete an infusion. Depending on the product and dosage, the prescriber will calculate the volume and how often to infuse the SCIg (called intervals). Larger intervals between infusions require larger amounts of product to achieve the required total SCIg dose to help the patient fight off infections (called a clinical response). It is important to remember that, just as every patient is unique, so every brand of SCIg is unique. Depending on which brand of SCIg is being used, the intervals and volume can be tailored to the unique medical needs and lifestyle of a particular patient. As long as the product’s total recommended dose is delivered every month, a patient can receive their brand-specific infusion as often as daily or, weekly, or biweekly.4,7
Some IVIg products can be used for both SCIg and IVIg. Others are for IVIg– or SCIg-use only.4 Because not every product or brand is right for every patient, you should talk to your healthcare provider to determine which SCIg product is right for you.
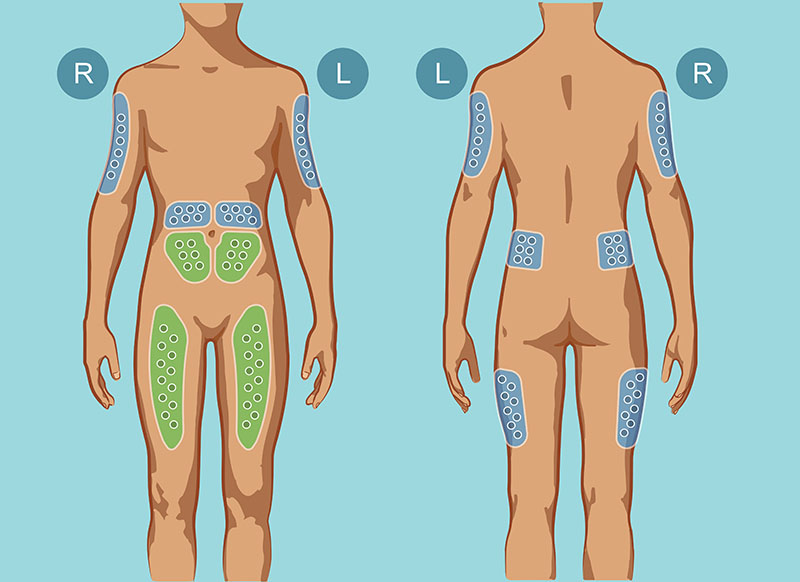
Figure 1: SCIg Infusion Sites
To administer SCIg independently, a patient (or caregiver) must first be taught how to infuse the SCIg correctly. They must also know what to expect during and after the infusion, including what to do in case of unwanted harmful side effects (called adverse events).9 Moreover, before a patient can be approved to self-administer SCIg, the patient should be prepared to demonstrate the correct self-administration techniques to a healthcare provider.7,9 It usually takes around 1 to 2 hours or less to infuse SCIg, depending on the volume and concentration of the product, and healthcare provider’s guidance.7,8
SCIg has been considered a safe and effective alternative to IVIg since the 1980s in Europe and since 2006 in the US.7,10 While SCIg is mostly well tolerated, the biologic can associated with mild and moderate local reactions, including redness, irritation, pain, and swelling at the infusion site.4,9 The incidence of local reactions with SCIg, although high (experienced in around 75% of patients), typically decreases over time.4,7 Serious systemic reactions, on the other hand, rarely occur with SCIg infusions (in less than 5% of patients).4,7 Most systemic reactions can be minimized using established mitigation or relief strategies, prescribed pre-medication, and/or pre-hydration.4 Mild-to-moderate systemic reactions to SCIg can include headache, fever, or muscle ache.11
Both patients and doses are carefully monitored over time. Doses are adjusted to minimize the frequency and severity of infections and adverse events for the patient, while IgG levels are monitored to correlate with clinical outcomes and the desired result of keeping the patient in good health.8 Because SCIg is usually infused regularly and often, IgG levels with SCIg therapy tend to remain constant.8
There are many options available with SCIg to help improve the infusion experience, including the number of needle-sticks and infusion sites, volume infused per site, needle width and length, and pump type. For example, if the number of needle sticks is a concern, it can be reduced by increasing the volume infused at each site. If higher volumes are not well tolerated, lower amounts can be administered on a daily schedule. If daily infusions are not an option, then fSCIg, which is given only once a month, might be the right choice. If a patient is experiencing discomfort at one site, another site can be used instead. Finally, needles and pumps can also be tried and tested until the right fit is found for an optimal infusion experience.8 Your healthcare provider can help familiarize you with the SCIg infusion options, so you can find a regimen that best suits your needs and lifestyle.