References:
1. Justiz Vaillant AA, Ramphul K. Immunoglobulin.[Updated 202 Mar 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from https://www.ncbi.nlm.nih.gov/books/NBK513460/
2. McCusker C, Upton J, Warrington R. Primary immunodeficiency. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):61-71.
3. Perez EE. Immunoglobulin use in immune deficiency and autoimmune disease states. Am J Manag Care. 2019 Jun;25(6 Suppl):S92-S97.
4. Chapel H, Prevot J, Gaspar HB, et al. Primary immune deficiencies – principles of care. Front Immunol. 2014;5(627):1-12.
5. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol. 2017;139(3):S1-S46.
6. Ness S. Differentiating characteristics and evaluating intravenous and subcutaneous immunoglobulin. Am J Care. 2019;25:S98-S104.
7. Barahona Afonso AF, Pires Joao, CM. The production process and biological effects of intravenous immunoglobulin. Biomolecules. 2016;15(6):1-20.
8. American Association of Blood Banks. Blood Donor Screening and Testing. AABB.org. http://www.aabb.org/advocacy/regulatorygovernment/donoreligibility/Pages/default.aspx. Accessed April 30, 2020.
9. US Food and Drug Administration. Blood & Blood Products. FDA.gov. https://www.fda.gov/vaccines-blood-biologics/blood-blood-products. Accessed March 29, 2020.
10. El-Ghariani K, Unsworth DJ. Therapeutic apheresis – plasmapheresis. Clin Med. 2006;6:343.
11. Skoda-Smith, Torgerson, Ochs. Subcutaneous immunoglobulin replacement therapy in the treatment of patients with primary immunodeficiency disease. Ther Clin Risk Manag. 2010;6:1-10
12. Kobrynski L. Subcutaneous immunoglobulin therapy: a new option for patients with primary immunodeficiency diseases. Biologics. 2012;6:277-287.
13. Hooper J. The history and evolution of immunoglobulin products and their clinical indications. J LymphoSign. 2015;2(4):181-194.
14. Aubert et al. History, extensive characterization and challenge of anti-tetanus serum from World War I: exciting remnants and deceived hopes : Centenarian IgGs lost their neutralization capacity. Immunol Res. Published online. 06 March 2020.
15. Hall WW. The US Navy’s war record with tetanus toxoid. Ann Intern Med. 1948;28(2):298-308.
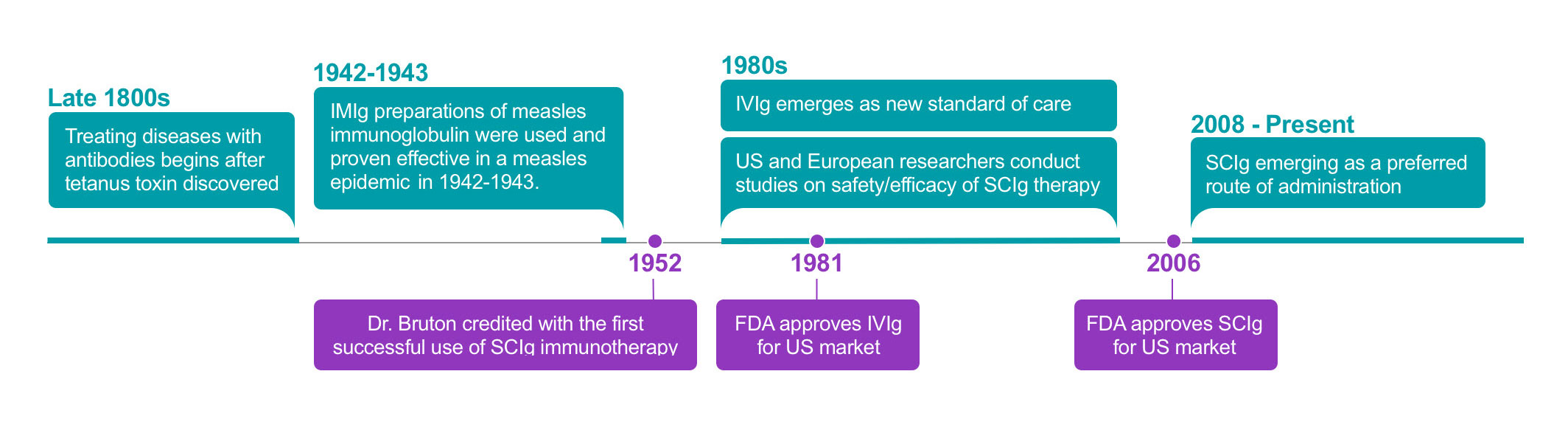
16. Bruton, OC. Agammaglobulinemia. Pediatrics. 1952;9(6)722-728.
17. Novaretti MC, Dinardo CL. Immunoglobulin: production, mechanisms of action and formulations. Rev Bras Hematol Hemoter. 2011:33(5):377-382.
18. Jolles, S. et al., New Frontiers in Subcutaneous Immunoglobulin Treatment. Biologics in Therapy. 2011: 1-15.